Regulatory Pharmacist
Suhitas Pharmaceuticals Inc.
Job Description
Benefits
Allowances
Transportation Allowance
Government Mandated Benefits
13th Month Pay, Pag-Ibig Fund, Paid Holidays, Philhealth, SSS/GSIS
Insurance Health & Wellness
Life Insurance, HMO
Time Off & Leave
Bereavement Leave, Sick Leave, Vacation Leave
REGULATORY AFFAIRS PHARMACIST
KEY FUNCTION
- Responsible for the company’s LTO application, renewal and variations.
- Responsible for handling product registration processes for Drugs, Medical Devices, Foods, Cosmetics including reviewing and preparing documentation and applications for submission to the FDA. Ensuring completeness, accuracy and adherence to regulatory guidelines.
- Prepare submissions of renewals of the CPR’s, monitor deadlines and ensure timely submission.
- Prepares submissions and communicate with distributors during the processing of CLIDP’s applications.
- Handles amendments / variations applications.
- Conduct thorough reviews of product labels (both primary and secondary packaging) for initial, CLIDP’s and renewal, verifying compliance with FDA labeling regulations, and proofreading all label content for accuracy and consistency before commercialization.
- Maintain a proactive follow-up with the FDA to track application status.
- Communicate with principals (manufacturers) regarding regulatory compliance, product issues, or clarifications needed for registration or variation processes.
- Ensure the company's Standard Operating Procedures (SOP’s) are up-to-date and aligned with the latest FDA regulations, conducting regular reviews and updates, and maintaining proper documentation for audit and compliance purposes.
- Responsible for monitoring and encoding of Electronic Drug Price Monitoring System (EDPMS) by the (DOH).
- Oversee the management of product-related concerns, including handling customer complaints, coordinating product recalls and withdrawals, and managing product distribution and returns within the subsidiary's scope of operations.
- Responsible for managing IPO applications and handling all related tasks and activities.
- Manage and securely maintain all documentation, including Certificates of Product Registration (CPRs) and Licenses to Operate (LTOs), ensuring their proper storage, accessibility, and confidentiality.
- Perform other duties as assigned by superiors.
Ensure compliance with regulatory requirements for drug products.
- Review and submit regulatory documents to health authorities.
- Collaborate with cross-functional teams for product development.
- Stay updated on changes in regulations and guidelines.
- Provide guidance on regulatory strategies and risk assessments.
- Conduct training sessions on regulatory compliance.
- Educational Qualifications: Bachelor’s degree in Pharmacy or a related field.
- Experience Level: 3-5 years of experience in regulatory affairs within the pharmaceutical industry.
- Skills and Competencies: Strong understanding of regulatory guidelines, excellent communication skills, and critical thinking ability.
- Working Conditions: Office environment with potential for remote work; may involve occasional travel.
- Qualities and Traits: Detail-oriented, proactive, adaptable, and strong ethical standards.
JOB REQUIREMENTS
· Licensed Pharmacist with 2-3 years of related experience in the related field
· In-depth knowledge of pharmaceutical regulations, guidelines, and submission procedures.
· Strong understanding of drug development, manufacturing processes, and quality standards.
· Excellent communication and interpersonal skills.
· Attention to detail and strong organizational abilities.
· Ability to interpret and analyze regulatory documents and data.
Laila Villapando
Admin SupervisorSuhitas Pharmaceuticals Inc.
Active within seven days
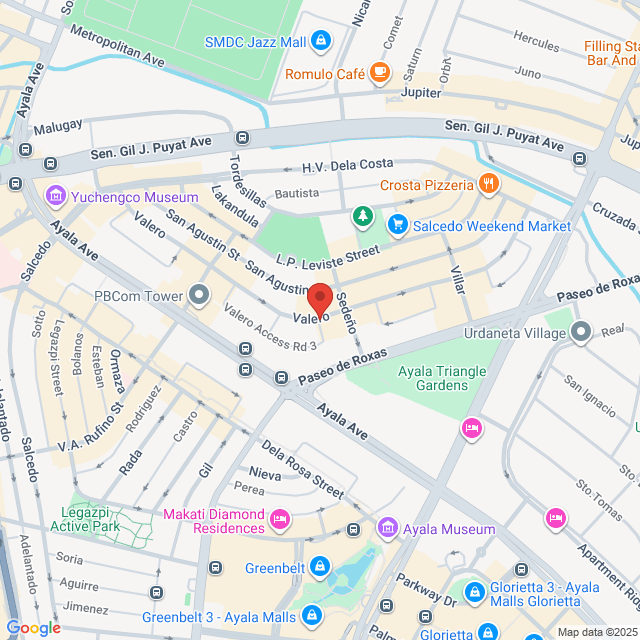
Working Location
Unit 1104 11Floor, 139 Valero, 139 Valero, Makati, 1227 Kalakhang Maynila, Philippines
Posted on 21 May 2025
Explore similar jobs
View more similar jobsRegulatory Affairs Pharmacist
 Allegens Healthcare Philippines Inc.
Allegens Healthcare Philippines Inc.Negotiable
On-site - Makati1-3 Yrs ExpBachelorFull-time

Nguyen Tuyet NuHR Manager
Regulatory Pharmacist
 Raya Regenerative and Preventive Medicine
Raya Regenerative and Preventive Medicine£386-515[Monthly]
On-site - Makati<1 Yr ExpBachelorFull-time

Justine GamboaHR Associate
Regulatory Pharmacist
 Orange Biotec Inc.
Orange Biotec Inc.£386-515[Monthly]
On-site - Makati3-5 Yrs ExpBachelorFull-time

Laila VillapandoHR SUPERVISOR
Regulatory Pharmacist
 Ambica International Corporation
Ambica International Corporation£257-386[Monthly]
On-site - PasayNo Exp RequiredBachelorFull-time
Race SeldaRecruitment Supervisor
Regulatory Pharmacist
 Main Source Manufacturing and Trading Corp.
Main Source Manufacturing and Trading Corp.£257-322[Monthly]
On-site - Caloocan1-3 Yrs ExpBachelorFull-time

MAIN SOURCE MANUFACTURING AND TRADING CORPHR Manager
Sign In to Chat with Boss
Bossjob Safety Reminder
If the position requires you to work overseas, please be vigilant and beware of fraud.
If you encounter an employer who has the following actions during your job search, please report it immediately
- withholds your ID,
- requires you to provide a guarantee or collects property,
- forces you to invest or raise funds,
- collects illicit benefits,
- or other illegal situations.

